mass of electron amu|4.4: The Properties of Protons, Neutrons, and Electrons : Cebu Peb 12, 2019 — The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. Electrons have an electric . The December 2023 PNP Entrance Exam (PNPE) results, including the official list of passers and topnotchers are out! The announcement is made by the National Police Commission (NAPOLCOM) thru its official website, 2 months after the schedule of exams. For the complete list of passers, kindly check the details below:
mass of electron amu,Mass of electron in amu (atomic mass unit): The mass of an electron in amu is determined by the mass of an electron ( in Kg ) divided by the mass of 1 amu . Mass of electron = 9 . 109 × 10 - 31 KgElectron mass m e = 9.109 ×10 −31 Kilograms. The electron mass value can .
Peb 12, 2019 — The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. Electrons have an electric .Mar 22, 2019 — Learn about the electron mass, a fundamental constant that is the mass of a stationary electron. Find out the value of electron mass in different units, such as amu, eV, kg, .Hul 29, 2022 — Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and interpret .In particle physics, the electron mass (symbol: m e) is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics.
The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. Electrons have an electric charge of \( .Learn how to define and calculate the atomic mass unit (amu) and the average atomic mass of elements based on their isotopic abundances. The mass of an electron is about 0.00055 amu, .
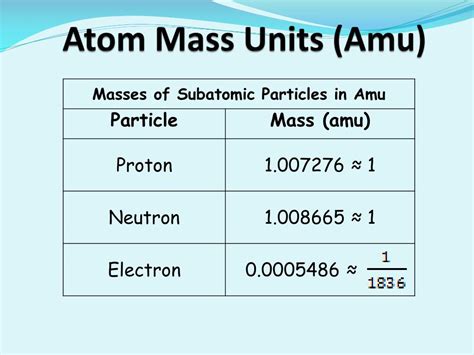
atomic mass unit (AMU), in physics and chemistry, a unit for expressing masses of atoms, molecules, or subatomic particles. An atomic mass unit is equal to 1/12 the mass of a single atom of carbon-12, the most abundant isotope of carbon, .The atomic mass unit (u or amu) is a relative unit based on a carbon-12 atom with six protons and six neutrons, which is assigned an exact value of 12 amu's (u's). This is the standard unit .Atomic mass unit (AMU), in physics and chemistry, a unit for expressing masses of atoms, molecules, or subatomic particles. An atomic mass unit is equal to 1 12 the mass of a single atom of carbon-12, the most abundant isotope of carbon, .The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (\(\text{amu}\)) is defined as one-twelfth the mass of a carbon-12 atom. Atomic mass units (\(\text{amu}\)) are useful, because, as you can see, the mass of a proton and the mass of a neutron are almost exactly \(1\) in this unit system.Neutrons are relatively heavy particles with no charge and a mass of 1.0087 amu. Electrons are light particles with a charge of 1− and a mass of 0.00055 amu. The number of protons in the nucleus is called the atomic number (Z) .Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 × × 10 −23 g, and an electron has a charge of less than 2 × × 10 −19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the unified atomic mass unit (u) and the .All isotopes of an element have the same number of protons and electrons, which means they exhibit the same chemistry. . This is the standard unit for atomic or molecular mass, and 1 amu is thus 1/12 th the mass of a 12 C atom. This is obviously very small. 1 amu = 1.66054x10-27 Kg = 1.66054x10-24 g.
mass of electron amuAs a result, a neutral atom must have an equal number of protons and electrons. The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom ; This page is shared under a CK-12 license and was authored, remixed, .Hun 30, 2019 — In chemistry, an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon-12.It is a unit of mass used to express atomic masses and molecular masses.When the mass is expressed in AMU, it roughly reflects the sum of the number of protons and neutrons in the atomic nucleus (electrons have so much less .The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute the bulk of the mass of atoms. . The relative masses of atoms are reported using the atomic mass unit (amu), which is defined as one-twelfth of the mass of one atom of carbon-12, with 6 protons, 6 neutrons, and 6 electrons.

The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. . As a result, a neutral atom must have an equal number of protons and electrons. The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom; 4.4: The .Abr 26, 2024 — The atomic mass is the mass of an atom, including its protons, neutrons, and electrons. However, because an electron is 1,836 times less massive than a proton, electrons account for an insignificant amount of mass of the atom in most atoms.. While you can state the atomic mass in kilograms, it is more usual to express it in atomic mass units (u \mathrm{u} u), .Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2 × 10 −23 g, and an electron has a charge of less than 2 × 10 −19 C (coulomb). When describing the properties of tiny objects such as atoms, we use appropriately small units of measure, such as the atomic mass unit (amu) and the .

The invariant mass of an electron is approximately 9.109 × 10 −31 kg, [79] or 5.489 × 10 −4 Da. Due to mass–energy equivalence, this corresponds to a rest energy of 0.511 MeV (8.19 × 10 −14 J). The ratio between the mass of a .4.4: The Properties of Protons, Neutrons, and ElectronsThe invariant mass of an electron is approximately 9.109 × 10 −31 kg, [79] or 5.489 × 10 −4 Da. Due to mass–energy equivalence, this corresponds to a rest energy of 0.511 MeV (8.19 × 10 −14 J). The ratio between the mass of a .Set 2, 2023 — Mass of Electron in KG and Mev. Electron weighs around 9.1091031 kilogrammes or 5.486104 daltons. The mass of an electron corresponds to an energy of around 8.1871014 joules, or about 0.5110 MeV. Mass of Electron in AMU. Mass of electron is 0. 0 0 0 5 5 amu. Mass of Electron is Equal to. J.J. Thomson, an English physicist, discovered the .
The atomic mass (m a or m) is the mass of an atom.Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1 Da is defined as 1 ⁄ 12 of the mass of a free carbon-12 atom at rest in its ground state. [1] The protons and neutrons of the nucleus account for .Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams, which scientists define as one atomic mass unit (amu) or one Dalton. Each electron has a negative charge (-1) equal to the positive charge of a proton (+1). Neutrons are uncharged particles found within the nucleus.mass of electron amu 4.4: The Properties of Protons, Neutrons, and ElectronsMay 20, 2018 — The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. . As a result, a neutral atom must have an equal number of protons and electrons. The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom; 4.4: The .The atomic mass unit (amu) was not standardized against hydrogen, but rather, against the 12 C isotope of carbon (amu = 12). Thus, the mass of the hydrogen atom (1 H) is 1.0080 amu, and the mass of an oxygen atom (16 O) is 15.995 amu. Once the masses of atoms were determined, the amu could be assigned an actual value: 1 amu = 1.66054 x 10-24 .
The atomic mass unit (amu) was not standardized against hydrogen, but rather, against the 12 C isotope of carbon (amu = 12). Thus, the mass of the hydrogen atom (1 H) is 1.0080 amu, and the mass of an oxygen atom (16 O) is 15.995 amu. Once the masses of atoms were determined, the amu could be assigned an actual value: 1 amu = 1.66054 x 10-24 .
mass of electron amu|4.4: The Properties of Protons, Neutrons, and Electrons
PH0 · What is the mass of an electron in amu? Chemistry Q&A
PH1 · In atomic mass units, what is the mass of an electron?
PH2 · Electron mass
PH3 · Electron Mass
PH4 · Atomic mass unit
PH5 · 5.6 Atomic Mass – Enhanced Introductory College Chemistry
PH6 · 4.4: The Properties of Protons, Neutrons, and Electrons
PH7 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH8 · 2.2: Atomic Number, Mass Number, and Atomic Mass Unit
PH9 · 1.9: The Properties of Protons, Neutrons, and Electrons